Spontaneous Void Spot Assay
Used to assess spontaneous voiding in unrestrained mice
Cystometry
Used to assess bladder pressure associated with filling and emptying
Uroflometry
Used to assess spontaneous urination in unrestrained mice
24-hour Urine Collection
Used to measure food and water intake and urine output. Urine is instantly preserved by freezing and stored for chemical, proteomic, metabolomic, genomic, or microbiomic analysis
Bladder Muscle Strip Contraction/Relaxation Assay
Used to assess in vitro muscle contractile or relaxation responses to pharmacological or electrical stimuli
SPONTANEOUS VOID SPOT ASSAY (VSA)
Overview Video
The spontaneous VSA is performed to assess spontaneous voiding in unrestrained mice
Strengths
- Non-invasive
- Can be repeated in the same mouse multiple times
- Inexpensive
- High throughput
- Reproducible between labs
Limitations
- Results are impacted by age, gender, strain and other factors
- Fullness of bladder at start is not controlled
- Unknown correlation to cystometrogram
- Affected by behavior
Equipment

Standard Operating Protocol
Void Whizzard Analysis Software
(free download and manual)
Minimal Information for Publishing VSA Results
- Refer to method as ‘spontaneous void spot assay’ to ensure consistency in future literature searches
- Feed and water type
- Mouse strain, sex, and age
- Filter paper product number, shape, and size
- Housing (single vs. group)
- Whether mice were acclimated to testing cage prior to analysis
- Time of day and length of monitoring period
- List analysis tool and settings
References
1971. Ralls K. Mammalian scent marking. Science 171: 443-449.
1973. Desjardins C, Maruniak JA and Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939-941.
1974. Maruniak JA, Owen K, Bronson FH, and Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav 12: 1035-1039.
1975. Maruniak JA, Owen K, Bronson FH, and Desjardins C. Urinary marking in female house mice: effects of ovarian steroids, sex experience, and type of stimulus. Behav Biol 13: 211-217.
1981. Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L and Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci USA 78: 5817-5820.
1985. Kimura T and Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Horm Behav 19: 64-70.
1987. Hurst JL. The functions of urine marking in a free-living population of house mice, Mus domesticus Rutty Animal Behaviour 35: 1433-1442.
1991. Stevens G, Joiner M, Joiner B, Johns H and Denekamp J. Early detection of damage following bilateral renal irradiation in the mouse. Radiother Oncol 20: 124-131.
1991. Stewart FA, Lundbeck F, Oussoren Y and Luts A. Acute and late radiation damage in mouse bladder: a comparison of urination frequency and cystometry. Int J Radiat Oncol Biol Phys 21: 1211-1219.
1993. Lundbeck F, Oussoren Y and Stewart FA. Early and late damage in the mouse bladder after radiation combined with cyclophosphamide or cisplatinum, evaluated by two different functional assays. Acta Oncol 32: 679-687.
1999. Lumley LA, Sipos ML, Charles RC, Charles RF, and Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav 67: 769-775.
2000. Nevison CM, Barnard CJ, Beynon RJ and Hurst JL. The consequences of inbreeding for recognizing competitors. Proc Biol Sci 267: 687-694.
2001 Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A and Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature 414: 631-634.
2007. Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, and Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453-3462.
2008. Arakawa H, Arakawa K, Blanchard DC and Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in c57bl/6j mice. Behav Brain Res 190: 97-104.
2008. Arakawa H, Blanchard DC, Arakawa K, Dunlap C and Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev 32: 1236-1248.
2008. Cornelissen LL, Misajet B, Brooks DP, and Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140: 53-58.
2008. Hodges SJ, Zhou G, Deng F, Aboushwareb T, Turner C, Andersson K, Santago P, Case D, Sun T and Christ GJ. Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin ii knockout mice. J Urol 179: 2046-2051.
2008. Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K and Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27: 548-552.
2009. Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, and Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139: 61-72.
2011. Rasmussen S, Miller MM, Filipski SB and Tolwani RJ. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci 50: 479-483.
2013. Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV and Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate 73: 1123-1133.
2013. Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, and Hill WG. Loss of beta1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. Faseb J 27: 1950-1961.
2013. Lee SH, Lysiak JJ and Steers WD. Bladder and urethral function in a mouse model of cavernous nerve injury. Neurourol Urodyn 32: 1038-1043.
2013. Lehmann ML, Geddes CE, Lee JL, and Herkenham M. Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice. PLoS One 8: e69822.
2013. Negoro H, Kanematsu A, Matsuo M, Okamura H, Tabata Y, Ogawa O. Development of diurnal micturition pattern in mice after weaning. J Urol 189: 740-746.
2014. Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C and Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol (Lond) 592: 537-549.
2014. Huang Y, Daneshgari F and Liu G. Successful induction of stress urinary incontinence in mice by vaginal distension does not depend on the estrous cycle. Urology 83: 958.e1-6.
2014. Mucignat-Caretta C, Cavaggioni A, Redaelli M, Da Dalt L, Zagotto G and Gabai G. Age and isolation influence steroids release and chemical signaling in male mice. Steroids 83: 10-16.
2014. Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, and Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296-1307.
2015. Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, and Peters LL. Aging Research Using Mouse Models. Curr Protoc Mouse Biol 5: 95-133.
2015. Biallosterski BT, Prickaerts J, Rahnama’i MS, de Wachter S, van Koeveringe GA, and Meriaux C. Changes in voiding behavior in a mouse model of Alzheimer’s disease. Front Aging Neurosci 7: 160.
2015. Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML, and Hill WG. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369-1378.
2015. Guo C, Kaneko S, Sun Y, Huang Y, Vlodavsky I, Li X, Li ZR, and Li X. A mouse model of urofacial syndrome with dysfunctional urination. Hum Mol Genet 24: 1991-1999.
2015. Keil KP, Abler LL, Altmann HM, Wang Z-Y, Wang P, Ricke WA, Bjorling DE, Vezina CM. Impact of a folic acid enriched diet on prostate and urinary function in mice susceptible to urinary dysfunction. Amer J Physiol-Renal 308:F1431-1443.
2015. Mann EA, Alam Z, Hufgard JR, Mogle M, Williams MT, Vorhees CV, and Reddy P. Chronic social defeat, but not restraint stress, alters bladder function in mice. Physiol Behav 150: 83-92.
2015. Nicholson TM, Moses M, Uchtmann K, Keil KP, Bjorling DE, Vezina CM, Wood RW, Ricke WA. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 193: 722-729.
2015. Ricke WA, Lee CW, Clapper TR, Schneider AJ, Moore RW, Keil KP, Abler LL, Wynder JL, López Alvarado A, Beaubrun I, Vo J, Bauman TM, Ricke EA, Peterson RE, and Vezina CM. In utero and Lactational TCDDExposure Increases Susceptibility to Lower Urinary Tract Dysfunction in Adulthood. Toxicol Sci 150: 429–440.
2016. Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, and Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn 35(2):192-198.
2018. Hill WG, Zeidel ML, Bjorling DE, Vezina CM. The void spot assay: Recommendations on the use of a simple micturition assay for mice. Am J Physiol Renal Physiol 315:F1422-F1429.
CYSTOMETRY
Overview Video
Strengths
- In vivo assessment of bladder function
- Generates significant amount of data
Limitations
- Effects of catheter placement on bladder function and structure
- Rate of filling differs from normal bladder filling
- Affected by age, strain, gender
Equipment
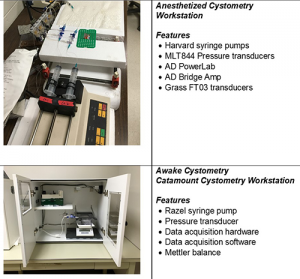
Standard Operating Protocol — Anesthetized_Cystometry
Standard Operating Protocol — Awake_Cystometry
Minimal Information for Publishing Cystometry Results
Anesthetized Cystometry
- Age, gender and strain of mice
- Amount and type of anesthetic
- Route of administration
- Size of cystostomy catheter (diameter)
- Description of transducer and data collection equipment
- Type of fluid infused into the bladder
- Rate of infusion
- Duration of equilibration prior to data collection
- Criteria for selection of tracing used for analysis
- Technique of euthanasia
- Analysis of tracing (separate section)
Awake Cystometry
- Age, gender and strain of mice
- Amount and type of anesthetic to place cystostomy catheter
- Route of administration
- Size of cystostomy catheter (diameter)
- Where catheter exited and how protected
- Time between catheter placement and testing
- How tubing connected to system
- Type of fluid infused into the bladder
- Rate of infusion
- Duration of equilibration prior to data collection
- Criteria for selection of tracing used for analysis
- Technique of euthanasia (if performed)
- Analysis of tracing (separate section)
Analysis_of_Cystometry_Tracings
References
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167.
Andersson KE, Soler R, Füllhase C. Rodent models for urodynamic investigation. Neurourol Urodyn 2011;30:636.
Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 2002;5:856.
Bjorling DE, Wang Z, Vezina CM, et al. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol. 2015;308:F1369.
Cornelissen LL, Misajet B, Brooks DP, Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 2008;140:53.
Dolber PC, Jin H, Nassar R, et al. The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice. Neurourol Urodynam 2013:34:72.
Fry CH, Daneshgari F, Thor K, et al. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn 2010;29:603.
Girard BM, Malley SE, Mathews MM, et al. Intravesical PAC1 Receptor Antagonist, PACAP, Reduces Urinary Bladder Frequency and Pelvic Sensitivity in NGF-OE Mice. J Mol Neurosci 2016;59:290.
Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats—effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn 2008;27:88.
Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn 2010;29:1344.
Smith PP, DeAngelis A, Kuchel GA.Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol 2012;302:R577.
Streng T, Hedlund P, Talo A, et al. Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 2006;97:1094.
Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 2008;139:158.
Yu W, Ackert-Bicknell C, Larigakis JD, et al. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 2014;306:F1296.
UROFLOMETRY
Overview
Uroflowmetry is performed to determine the frequency and volume of spontaneous urination in unrestrained mice. Information on creating the system used and obtaining the software can be found here.
Strengths
- Can compare urine flow rates and volumes
- Video capability, when available, will allow characterization of void pattern (dribbling, drops, full stream)
- Correlate urine flow rate with characterization of void
Limitations
- Urine may cling to grate
- Providing mice sweetened water will artificially increase urine output
- Possible that some strains of mice may not void within 2 hours; however, increased water intake decreases the likelihood of this
Standard Operating Protocol — Uroflow_Test
Minimal Information for Publishing Uroflometry Results
- Two days prior to testing, mice are provided drinking water containing 3% glucose and 0.125% saccharin ad libitum to increase urine output
- Videocameras are focused on the underside of the metabolic cage
- Mice are placed in metabolic cages positioned above Mettler-Toledo precision balances (Columbus, OH)
- Data are captured from the balances and videocameras using a subprogram written using LabVIEW
- Urine flow is determined and the shape of the void curve is correlated with the pattern of urine flow to provide qualitative and quantitative information regarding voiding
References
Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. (2012) Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 153:5556-65.
Wood R, Eichel L, Messing EM, Schwarz E. (2001) Automated noninvasive measurement of cyclophosphamide-induced changes in murine voiding frequency and volume. J Urol. 165:653-9.
Leung YY, Schwarz EM, Silvers CR, Messing EM, Wood RW. (2004) Uroflow in murine urethritis. Urology. 64:378-82.
Wood RW, Baggs RB, Schwarz EM, Messing EM. (2006) Initial observations of reduced uroflow in transgenic adenocarcinoma of murine prostate. Urology. 67:1324-8. Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. (2012) Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 153:5556-65.
24-HOUR URINE COLLECTION
Overview
24-hour Urine Collection is performed to measure food and water intake and urine output. Urine is instantly preserved by freezing and stored for chemical, proteomic, metabolomic, genomic, or microbiomic analysis.

Strengths
- Determines 24 hour urine output
- Measures water and food intake
- Freezes urine upon collection for subsequent analysis
- Measures fecal output
Limitations
- Does not determine pattern of urination
- Requires acclimation to cages
- Some strains of mice may be reluctant to drink, eat, or void
Equipment

Standard Operating Protocol
Minimal Information for Publishing 24 Hour Metabolic Cage Results
- Mice habituated to cages by placement in cages for 2-4 hours per day for 2 days prior to testing
- Mice allowed access to standard chow and water to which they are accustomed; food and water intake monitored
- Mice placed in metabolic cage with optional freezing unit to freeze urine immediately upon collection
- Feces separated from urine and fecal output can be measured if desired (feces also frozen upon collection)
References
Columbus Instruments. Relevant material in Waste Collection section.
BLADDER MUSCLE STRIP CONTRACTION/RELAXATION ASSAY
Tissue baths are used to assess muscle contractile or relaxation responses to pharmacological or electrical stimuli
Strengths
- Specifically evaluates detrusor contraction or relaxation in response to various stimuli
- Can evaluate influence of urothelium on response by leaving intact or removing
Limitations
- Lack of systemic input from circulation, lymphatics, nervous system, and adjacent tissues
- Dimensions and orientation of strips of tissue studied may not faithfully replicate forces applied and generated in situ
Standard Operating Protocol — Tissue_Baths
Minimal Information for Publishing Tissue Bath Assay Results
- Method of euthanasia
- Lapse of time between euthanasia and harvesting tissues
- Orientation of bladder strips removed (longitudinal or circumferential)
- Urothelium left intact or removed
- Composition of balanced salt solution in baths
- Duration of equilibration
- Amount of tension applied to tissues during equilibration and testing (prior to applying stimuli)
- Order of testing (i.e., what are tissues first exposed to followed by the progression of tests)
- Standardization of contractile response (contractile force can be reported in grams or millinewtons but must be normalized; typically normalized to maximal contraction in response to KCl or carbachol; maximum contractile response considered 100% and response to other stimuli calculated relative to this; can further normalize to tissue weight)
- Results typically reported as EC10 (the concentration or rate of stimulation that results in 10% maximal contraction) or EC50 (the concentration or rate of stimulation that results in 50% maximal contraction)
- Effects of stimuli evaluated by comparison of EC10 or EC50
References
Bjorling DE, et al. Response of the isolated guinea pig bladder to exogenous and endogenous leukotrienes. J Urol 2994;152:1281.
Busser BW, et al. Lipopolysaccharide upregulates bradykinin 1 receptors in the isolated mouse bladder. J Urol 1998;160:2267.